REDONNA was a single-blinded, two-arm clinical trial of a prescribing Portrait for eligible family physicians in British Columbia, Canada. Exposure
Connecting professionals to promote interdisciplinary trauma-focused therapy
Connecting professionals can promote interdisciplinary trauma-focused therapy and return-to-work support for clients with work-related PTSD. This blog
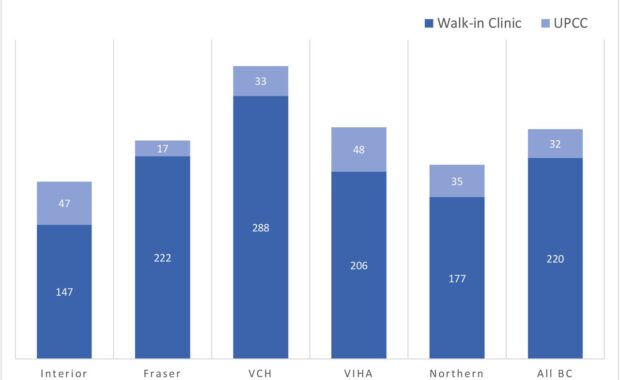
Comparing Walk-in Clinics and Urgent Primary Care Centers
Walk-in clinics are non-hospital based primary care facilities that are designed to operate without appointments and provide increased healthcare
Group therapy alleviates psychological distress in prostate cancer
Emerging evidence that group therapy helps men with prostate cancer.
I’m almost opioid-a-phobic: Family medicine residents perceive
Over the past two decades, Canadians’ use of opioid analgesics has substantially grown, making the nation the second-largest user of opioid analgesics
Filling the substance use treatment gap requires better education and research training
While education is unlikely the ultimate filling for the substance use treatment gap, it is one that can be hardly overlooked and
How can people with opioid use disorder get better virtual care?
Virtual care is the new normal around the globe. The emergence of COVID-19 introduced a dual public health emergency in British Columbia. The province
Dennis McCarty receives prestigious Kentucky award 2022
Dennis McCarty, Ph.D., an Emeritus Professor in the School of Public Health at the Portland State University and Oregon Health & Science
Why inequality hinders effective pain relief for opioid naive people
Equitable access to care is problematic; some people get it, most are left out. The REDONNA study (https://doi.org/10.1016/j.cct.2021.106462) began